Which Of These Is Not An Important Structural Feature On The Surface Membrane Of An Animal Cell?
Like all other cellular membranes, the plasma membrane consists of both lipids and proteins. The primal structure of the membrane is the phospholipid bilayer, which forms a stable barrier between two aqueous compartments. In the case of the plasma membrane, these compartments are the inside and the outside of the cell. Proteins embedded within the phospholipid bilayer carry out the specific functions of the plasma membrane, including selective transport of molecules and cell-prison cell recognition.
The Phospholipid Bilayer
The plasma membrane is the most thoroughly studied of all cell membranes, and it is largely through investigations of the plasma membrane that our current concepts of membrane construction have evolved. The plasma membranes of mammalian red blood cells (erythrocytes) have been particularly useful as a model for studies of membrane structure. Mammalian red blood cells do not contain nuclei or internal membranes, and so they correspond a source from which pure plasma membranes can be easily isolated for biochemical analysis. Indeed, studies of the red blood jail cell plasma membrane provided the first evidence that biological membranes consist of lipid bilayers. In 1925, two Dutch scientists (E. Gorter and R. Grendel) extracted the membrane lipids from a known number of red blood cells, corresponding to a known surface area of plasma membrane. They then determined the surface area occupied by a monolayer of the extracted lipid spread out at an air-h2o interface. The surface area of the lipid monolayer turned out to be twice that occupied by the erythrocyte plasma membranes, leading to the determination that the membranes consisted of lipid bilayers rather than monolayers.
The bilayer structure of the erythrocyte plasma membrane is clearly evident in high-magnification electron micrographs (Figure 12.1). The plasma membrane appears as ii dumbo lines separated by an intervening infinite—a morphology oft referred to as a "railroad track" advent. This image results from the binding of the electron-dense heavy metals used as stains in transmission electron microscopy (see Chapter 1) to the polar head groups of the phospholipids, which therefore appear every bit dark lines. These dense lines are separated by the lightly stained interior portion of the membrane, which contains the hydrophobic fat acid chains.

Figure 12.one
Bilayer structure of the plasma membrane. Electron micrograph of a homo red blood cell. Note the railroad track advent of the plasma membrane. (Courtesy of J. David Robertson, Duke University Medical Center.)
As discussed in Chapter 2, the plasma membranes of brute cells contain four major phospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin), which together account for more than half of the lipid in most membranes. These phospholipids are asymmetrically distributed between the 2 halves of the membrane bilayer (Figure 12.two). The outer leaflet of the plasma membrane consists mainly of phosphatidylcholine and sphingomyelin, whereas phosphatidylethanolamine and phosphatidylserine are the predominant phospholipids of the inner leaflet. A fifth phospholipid, phosphatidylinositol, is likewise localized to the inner half of the plasma membrane. Although phosphatidylinositol is a quantitatively minor membrane component, information technology plays an important role in prison cell signaling, as discussed in the next chapter. The head groups of both phosphatidylserine and phosphatidylinositol are negatively charged, so their predominance in the inner leaflet results in a net negative charge on the cytosolic face of the plasma membrane.
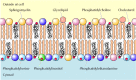
Figure 12.2
Lipid components of the plasma membrane. The outer leaflet consists predominantly of phosphatidylcholine, sphingomyelin, and glycolipids, whereas the inner leaflet contains phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol. Cholesterol (more...)
In addition to the phospholipids, the plasma membranes of animal cells contain glycolipids and cholesterol. The glycolipids are constitute exclusively in the outer leaflet of the plasma membrane, with their carbohydrate portions exposed on the cell surface. They are relatively minor membrane components, constituting only about 2% of the lipids of nearly plasma membranes. Cholesterol, on the other manus, is a major membrane constituent of animal cells, being present in about the aforementioned molar amounts as the phospholipids.
Ii general features of phospholipid bilayers are critical to membrane role. Beginning, the construction of phospholipids is responsible for the basic function of membranes as barriers between two aqueous compartments. Because the interior of the phospholipid bilayer is occupied by hydrophobic fatty acid bondage, the membrane is impermeable to water-soluble molecules, including ions and virtually biological molecules. Second, bilayers of the naturally occurring phospholipids are gluey fluids, non solids. The fatty acids of most natural phospholipids have one or more than double bonds, which introduce kinks into the hydrocarbon bondage and make them difficult to pack together. The long hydrocarbon chains of the fatty acids therefore move freely in the interior of the membrane, so the membrane itself is soft and flexible. In addition, both phospholipids and proteins are free to diffuse laterally inside the membrane—a property that is critical for many membrane functions.
Because of its rigid ring construction, cholesterol plays a distinct part in membrane structure. Cholesterol will not grade a membrane past itself, but inserts into a bilayer of phospholipids with its polar hydroxyl grouping close to the phospholipid head groups (meet Effigy 12.2). Depending on the temperature, cholesterol has distinct effects on membrane fluidity. At high temperatures, cholesterol interferes with the movement of the phospholipid fatty acid chains, making the outer part of the membrane less fluid and reducing its permeability to small-scale molecules. At low temperatures, notwithstanding, cholesterol has the opposite result: Past interfering with interactions between fatty acid chains, cholesterol prevents membranes from freezing and maintains membrane fluidity. Although cholesterol is not nowadays in leaner, it is an essential component of animate being jail cell plasma membranes. Establish cells also lack cholesterol, just they incorporate related compounds (sterols) that fulfill a similar part.
Contempo studies suggest that not all lipids diffuse freely in the plasma membrane. Instead, discrete membrane domains announced to be enriched in cholesterol and the sphingolipids (sphingomyelin and glycolipids). These clusters of sphingolipids and cholesterol are thought to grade "rafts" that move laterally within the plasma membrane and may associate with specific membrane proteins. Although the functions of lipid rafts remain to be understood, they may play of import roles in processes such equally jail cell signaling and the uptake of extracellular molecules by endocytosis.
Membrane Proteins
While lipids are the fundamental structural elements of membranes, proteins are responsible for conveying out specific membrane functions. Most plasma membranes consist of approximately fifty% lipid and 50% poly peptide past weight, with the carbohydrate portions of glycolipids and glycoproteins constituting 5 to 10% of the membrane mass. Since proteins are much larger than lipids, this per centum corresponds to about one protein molecule per every 50 to 100 molecules of lipid. In 1972, Jonathan Singer and Garth Nicolson proposed the fluid mosaic model of membrane construction, which is now generally accustomed as the basic paradigm for the organization of all biological membranes. In this model, membranes are viewed as two-dimensional fluids in which proteins are inserted into lipid bilayers (Figure 12.3).

Figure 12.3
Fluid mosaic model of the plasma membrane. Integral membrane proteins are inserted into the lipid bilayer, whereas peripheral proteins are spring to the membrane indirectly by protein-protein interactions. Near integral membrane proteins are transmembrane (more...)
Singer and Nicolson distinguished ii classes of membrane-associated proteins, which they called peripheral and integral membrane proteins. Peripheral membrane proteins were operationally divers every bit proteins that dissociate from the membrane following treatments with polar reagents, such every bit solutions of extreme pH or high salt concentration, that do non disrupt the phospholipid bilayer. Once dissociated from the membrane, peripheral membrane proteins are soluble in aqueous buffers. These proteins are not inserted into the hydrophobic interior of the lipid bilayer. Instead, they are indirectly associated with membranes through protein-protein interactions. These interactions often involve ionic bonds, which are disrupted by extreme pH or high salt.
In contrast to the peripheral membrane proteins, integral membrane proteins tin be released only by treatments that disrupt the phospholipid bilayer. Portions of these integral membrane proteins are inserted into the lipid bilayer, so they tin can exist dissociated only by reagents that disrupt hydrophobic interactions. The nigh commonly used reagents for solubilization of integral membrane proteins are detergents, which are pocket-size amphipathic molecules containing both hydrophobic and hydrophilic groups (Figure 12.iv). The hydrophobic portions of detergents displace the membrane lipids and bind to the hydrophobic portions of integral membrane proteins. Because the other terminate of the detergent molecule is hydrophilic, the detergent-protein complexes are soluble in aqueous solutions.

Effigy 12.4
Solubilization of integral membrane proteins by detergents. Detergents (eastward.yard., octyl glucoside) are amphipathic molecules containing hydrophilic head groups and hydrophobic tails. The hydrophobic tails demark to the hydrophobic regions of integral membrane (more...)
Many integral proteins are transmembrane proteins, which span the lipid bilayer with portions exposed on both sides of the membrane. These proteins tin can be visualized in electron micrographs of plasma membranes prepared by the freeze-fracture technique (see Figure ane.35). In these specimens, the membrane is split and separates into its 2 leaflets. Transmembrane proteins are then apparent as particles on the internal faces of the membrane (Effigy 12.v).

Figure 12.5
Freeze-fracture electron micrograph of man red blood prison cell membranes. The particles in the membrane are transmembrane proteins. (Harold H. Edwards/Visuals Unlimited.)
The membrane-spanning portions of transmembrane proteins are usually α helices of xx to 25 hydrophobic amino acids that are inserted into the membrane of the endoplasmic reticulum during synthesis of the polypeptide chain (come across Figures ix.xi, 9.12, and 9.thirteen). These proteins are then transported in membrane vesicles from the endoplasmic reticulum to the Golgi apparatus, and from there to the plasma membrane. Saccharide groups are added to the polypeptide bondage in both the endoplasmic reticulum and Golgi appliance, then well-nigh transmembrane proteins of the plasma membrane are glycoproteins with their oligosaccharides exposed on the surface of the cell.
Studies of red blood cells have provided practiced examples of both peripheral and integral proteins associated with the plasma membrane. The membranes of human erythrocytes incorporate virtually a dozen major proteins, which were originally identified by gel electrophoresis of membrane preparations. Most of these are peripheral membrane proteins that have been identified as components of the cortical cytoskeleton, which underlies the plasma membrane and determines cell shape (run across Chapter 11). For case, the most abundant peripheral membrane protein of scarlet blood cells is spectrin, which is the major cytoskeletal protein of erythrocytes. Other peripheral membrane proteins of red blood cells include actin, ankyrin, and band iv.1. Ankyrin serves as the principal link between the plasma membrane and the cytoskeleton by binding to both spectrin and the integral membrane protein ring iii (run into Figure 11.xi). An additional link between the membrane and the cytoskeleton is provided by ring iv.1, which binds to the junctions of spectrin and actin, also as to glycophorin (the other major integral membrane poly peptide of erythrocytes).
The two major integral membrane proteins of red claret cells, glycophorin and band three, provide well-studied examples of transmembrane protein structure (Effigy 12.6). Glycophorin is a pocket-size glycoprotein of 131 amino acids, with a molecular weight of nearly 30,000, one-half of which is protein and one-half carbohydrate. Glycophorin crosses the membrane with a single membrane-spanning α helix of 23 amino acids, with its glycosylated amino-terminal portion exposed on the cell surface. Although glycophorin was one of the first transmembrane proteins to exist characterized, its precise office remains unknown. In contrast, the part of the other major transmembrane protein of red blood cells is well understood. This protein, originally known as band 3, is the anion transporter responsible for the passage of bicarbonate (HCO3 -) and chloride (Cl-) ions across the carmine blood cell membrane. The ring 3 polypeptide chain is 929 amino acids and is thought to have 14 membrane-spanning α-helical regions. Within the membrane, dimers of band 3 form globular structures containing internal channels through which ions are able to travel across the lipid bilayer.
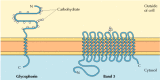
Figure 12.half-dozen
Integral membrane proteins of red claret cells. Glycophorin (131 amino acids) contains a single transmembrane α helix. It is heavily glyocosylated, with oligosaccharides attached to 16 sites on the extracellular portion of the polypeptide concatenation. (more...)
Because of their amphipathic character, transmembrane proteins have proved difficult to crystallize, every bit required for three-dimensional structural analysis by X-ray diffraction. The first transmembrane poly peptide to be analyzed by X-ray crystallography was the photosynthetic reaction middle of the bacterium Rhodopseudomonas viridis, whose structure was reported in 1985 (Figure 12.vii). The reaction centre contains three transmembrane proteins, designated L, Chiliad, and H (calorie-free, medium, and heavy) according to their credible sizes indicated by gel electrophoresis. The L and One thousand subunits each accept five membrane-spanning α helices. The H subunit has only a single transmembrane α helix, with the majority of the polypeptide chain on the cytosolic side of the membrane. The 4th subunit of the reaction centre is a cytochrome, which is a peripheral membrane protein jump to the complex past protein-protein interactions.

Figure 12.vii
A bacterial photosynthetic reaction center. The reaction center consists of three transmembrane proteins, designated L (cherry-red), Grand (yellow), and H (green). The L and M subunits each have five transmembrane α helices, whereas the H subunit has only (more...)
Although most transmembrane proteins span the membrane by α-helical regions, this is not always the case. A well-characterized exception is provided by the porins—a class of proteins that course channels in the outer membranes of some bacteria. Many leaner, including E. coli, have a dual membrane system in which the plasma membrane (or inner membrane) is surrounded by the cell wall and a singled-out outer membrane (Figure 12.eight). In contrast to the plasma membrane, the outer membrane is highly permeable to ions and small polar molecules (in the case of E. coli, with molecular weights up to 600). This permeability results from the porins, which form open aqueous channels through the lipid bilayer. As discussed in Affiliate 10, proteins related to the bacterial porins are also found in the outer membranes of mitochondria and chloroplasts.
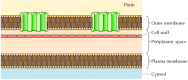
Figure 12.8
Bacterial outer membranes. The plasma membrane of some bacteria is surrounded past a jail cell wall and a distinct outer membrane. The outer membrane contains porins, which form open aqueous channels allowing the costless passage of ions and small molecules.
Structural assay has indicated that the porins practice non comprise hydrophobic α-helical regions. Instead, they cross the membrane as β barrels, in which 16 β sheets fold upwardly into a barrel-like structure enclosing an aqueous pore (Figure 12.9). The side bondage of polar amino acids line the pore, whereas side chains of hydrophobic amino acids interact with the interior of the membrane. The porin monomers acquaintance to class stable trimers, each of which contains iii open channels through which polar molecules can diffuse across the membrane.

Figure 12.ix
Structure of a porin monomer. Each monomer is a β barrel consisting of 16 antiparallel β strands (arrows). The acme end of the molecule faces the external medium. (From H. Nikaido, 1994. J. Biol. Chem. 269: 3905.)
In dissimilarity to transmembrane proteins, a variety of proteins (many of which bear equally integral membrane proteins) are anchored in the plasma membrane by covalently fastened lipids or glycolipids (Effigy 12.10). Members of one course of these proteins are inserted into the outer leaflet of the plasma membrane by glycosylphosphatidylinositol (GPI) anchors. GPI anchors are added to certain proteins that take been transferred into the endoplasmic reticulum and are anchored in the membrane by a C-final transmembrane region (see Figure 9.sixteen). The transmembrane region is broken as the GPI anchor is added, so these proteins remain attached to the membrane only by the glycolipid. Since the polypeptide bondage of GPI-anchored proteins are transferred into the endoplasmic reticulum, they are glycosylated and exposed on the surface of the cell following send to the plasma membrane.
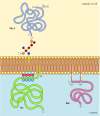
Figure 12.10
Examples of proteins anchored in the plasma membrane by lipids and glycolipids. Some proteins (e.k., the lymphocyte poly peptide Thy-one) are anchored in the outer leaflet of the plasma membrane by GPI anchors added to their C terminus in the endoplasmic reticulum. (more...)
Other proteins are anchored in the inner leaflet of the plasma membrane past covalently attached lipids. Rather than beingness candy through the secretory pathway, these proteins are synthesized on complimentary cytosolic ribosomes and and so modified by the addition of lipids. These modifications include the addition of myristic acid (a 14-carbon fatty acid) to the amino terminus of the polypeptide chain, the improver of palmitic acrid (16 carbons) to the side bondage of cysteine residues, and the addition of prenyl groups (xv or twenty carbons) to the side bondage of carboxy-terminal cysteine residues (meet Figures 7.29, 7.30, and 7.31). In some cases, these proteins (many of which deport as peripheral membrane proteins) are targeted to the plasma membrane by positively charged regions of the polypeptide chain besides as by the attached lipids. These positively charged protein domains may interact with the negatively charged head groups of phosphatidylserine on the cytosolic face of the plasma membrane. It is noteworthy that many of the proteins anchored in the inner leaflet of the plasma membrane (including the Src and Ras proteins illustrated in Figure 12.10) play of import roles in the transmission of signals from cell surface receptors to intracellular targets, every bit discussed in the next chapter.
Mobility of Membrane Proteins
Membrane proteins and phospholipids are unable to movement back and along betwixt the inner and outer leaflets of the membrane at an observable rate. Even so, because they are inserted into a fluid lipid bilayer, both proteins and lipids are able to diffuse laterally through the membrane. This lateral movement was start shown straight in an experiment reported by Larry Frye and Michael Edidin in 1970, which provided support for the fluid mosaic model. Frye and Edidin fused human and mouse cells in culture to produce human-mouse cell hybrids (Effigy 12.11). They then analyzed the distribution of proteins in the membranes of these hybrid cells using antibodies that specifically recognize proteins of homo and mouse origin. These antibodies were labeled with dissimilar fluorescent dyes, so the human and mouse proteins could exist distinguished by fluorescence microscopy. Immediately later on fusion, human and mouse proteins were localized to different halves of the hybrid cells. Notwithstanding, later on a brief period of incubation at 37°C, the human and mouse proteins were completely intermixed over the cell surface, indicating that they moved freely through the plasma membrane.

Figure 12.xi
Mobility of membrane proteins. Human and mouse cells were fused to produce hybrid cells. The distribution of cell surface proteins was and so analyzed using anti-human and anti-mouse antibodies labeled with different fluorescent dyes (red and green, respectively). (more...)
All the same, non all proteins are able to diffuse freely through the membrane. In some cases, the mobility of membrane proteins is restricted by their association with the cytoskeleton. For example, a fraction of band three in the cherry-red blood jail cell membrane is immobilized as a upshot of its clan with ankyrin and spectrin. In other cases, the mobility of membrane proteins may be restricted by their associations with other membrane proteins, with proteins on the surface of next cells, or with the extracellular matrix.
In contrast to blood cells, epithelial cells are polarized when they are organized into tissues, with different parts of the cell responsible for performing distinct functions. Consequently, the plasma membranes of many epithelial cells are divided into distinct apical and basolateral domains that differ in role and protein composition (Figure 12.12). For example, epithelial cells of the small intestine function to absorb nutrients from the digestive tract. The apical surface of these cells, which faces the intestinal lumen, is therefore covered by microvilli and specialized for nutrient assimilation. The basolateral surface, which faces underlying connective tissue and the blood supply, is specialized to mediate the transfer of absorbed nutrients into the circulation. In guild to maintain these distinct functions, the mobility of plasma membrane proteins must be restricted to the appropriate domains of the cell surface. At least part of the mechanism by which this occurs involves the formation of tight junctions (which are discussed later in this chapter) betwixt adjacent cells of the epithelium. These junctions not but seal the space between cells but besides serve as barriers to the move of membrane lipids and proteins. As a result, proteins are able to lengthened within either the apical or basolateral domains of the plasma membrane but are not able to cantankerous from one domain to the other.
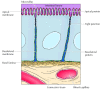
Figure 12.12
A polarized abdominal epithelial cell. The upmost surface of the cell contains microvilli and is specialized for absorption of nutrients from the abdominal lumen. The basolateral surface is specialized for the transfer of captivated nutrients to the underlying (more...)
The Glycocalyx
As already discussed, the extracellular portions of plasma membrane proteins are generally glycosylated. Likewise, the carbohydrate portions of glycolipids are exposed on the outer face of the plasma membrane. Consequently, the surface of the cell is covered by a saccharide glaze, known as the glycocalyx, formed past the oligosaccharides of glycolipids and transmembrane glycoproteins (Effigy 12.thirteen).

Effigy 12.13
The glycocalyx. An electron micrograph of intestinal epithelium illustrating the glycocalyx (arrows). (Don Fawcett/ Visuals Unlimited.)
Part of the role of the glycocalyx is to protect the cell surface. In addition, the oligosaccharides of the glycocalyx serve as markers for a diversity of cell-cell interactions. A well-studied example of these interactions is the adhesion of white blood cells (leukocytes) to the endothelial cells that line claret vessels—a process that allows the leukocytes to exit the circulatory organisation and mediate the inflammatory response in injured tissues. The initial step in adhesion between leukocytes and endothelial cells is mediated past a family unit of transmembrane proteins called selectins, which recognize specific carbohydrates on the cell surface (Figure 12.14). Two members of the selectin family (Due east-selectin and P-selectin), expressed past endothelial cells and platelets, demark to specific oligosaccharides expressed on the surface of leukocytes. A different selectin (Fifty-selectin) is expressed by leukocytes and recognizes an oligosaccharide on the surface of endothelial cells. The oligosaccharides exposed on the prison cell surface thus provide a fix of markers that help place the singled-out cell types of multicellular organisms.
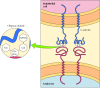
Figure 12.14
Binding of selectins to oligosaccharides. E-selectin is a transmembrane protein expressed by endothelial cells that binds to an oligosaccharide expressed on the surface of leukocytes. The oligosaccharide recognized by E-selectin contains North-acetylglucosamine (more than...)
Source: https://www.ncbi.nlm.nih.gov/books/NBK9898/
Posted by: franklinhoge1949.blogspot.com

0 Response to "Which Of These Is Not An Important Structural Feature On The Surface Membrane Of An Animal Cell?"
Post a Comment